Engineering self-assembling kinesin tracks
.K200AF(ATP100)-001-1.gif)
The microtubule (red) deposits kinesin (green) on the surface and is seen to leave a kinesin trail behind, which then detaches from the surface. Often, multiple microtubules will use the same kinesin track. Movie is at 20x speed.
One of the hallmarks of biological systems is their ability to assemble structures as needed. Structures often spontaneously disassemble once they have fulfilled their purpose, thus making it possible to recycle building blocks. I propose that this is a result of their building blocks being held in place by relatively weak interactions rather than extremely strong bonds which usually characterize the materials we use currently in engineered systems.
I created a proof-of-concept system in which microtubules would attract kinesin motors to the surface and deposit them as they were propelled along the surface by those kinesin motors. Thus, the kinesin trail would be assembled as needed by the microtubule. However, the kinesin motors are reversibly bound to the surface and thus after propelling the microtubule off of themselves, would be free to leave the surface. Thus, the microtubule acts as both a concentrator of kinesin motors towards the surface as well as a stabilizer for the kinesin-surface bond. I showed that a simple chemical model of this system is sufficient to capture the essence of its behavior, successfully predicting the tradeoff between the maximum velocity achieved by the propelled microtubule and the minimum number of kinesin motors necessary in the system.
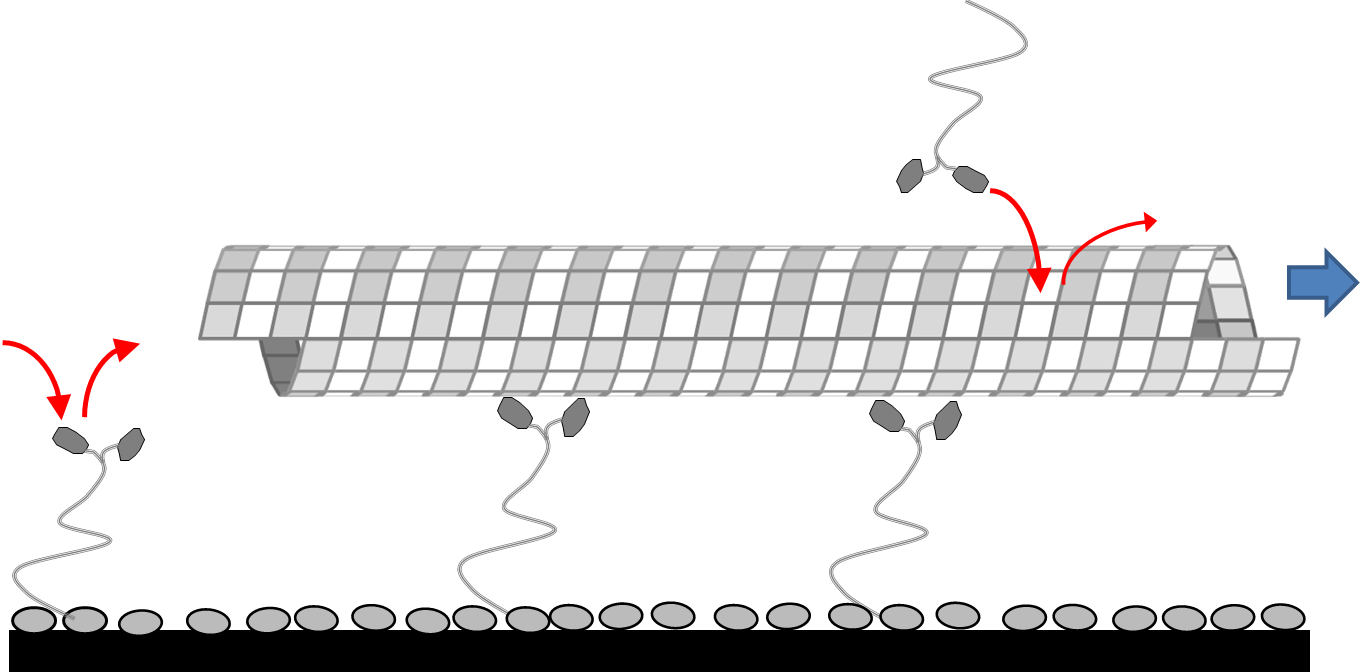
The microtubule acts to stabilize the bond between kinesin and surface, and thus is able to write its own track.
Self-assembling systems involving weaker bonds seem to be an interesting avenue of study. In cases where there is an abundance of resources, weak bonds between components in the system would allow for a constant replacement of parts, which would prevent defective components from building up. This is particularly important in biological tissues which are made of proteins which often have lifetimes on the order of days. Furthermore, in the case where there is an excess of building blocks, because the structure is self-assembled, additional components can be recruited when necessary allowing for material adaptability and healing. It is my hope that my experimental system will allow for the exploration of these capabilities.
For further reading, see A. T. Lam, S. Tsitkov, Y. Zhang, and H. Hess, Nano Letters (2018).